Module 64: STEREOISOMERS: enantiomers, diastereomers, chirality, stereocenters,R/S naming,
9th ed pseudo-asymmetric centers, Fischer projections
From this module and the lecture videos you should be able to:
- Identify whether two molecular representations are superposable or non-superposable. (64.01)
- Determine the relationship between two stereoisomers (enantiomers or diastereomers). (64.02)
- Identify chiral and achiral molecules using internal mirror planes and the inversion operation. (64.03)
- Know that enantiomers are chiral and diastereomers can be either chiral or achiral.
- Know the similarities/differences in physical properties of enantiomers / diastereomers.
- Identify stereocenters. Know that stereocenters are a prerequisite for a molecule being chiral, but not a guarantee that such a molecule will be chiral.
- Know the difference between stereocenter and an asymmetric center.
- Identify meso compounds.
- Determine the R/S nomenclature of asymmetric centers in molecules.
- Convert a Fisher projection to a line structure and vice versa, as well as determine R/S nomenclature for asymmetric centers in Fisher projections.
- Know racemic mixtures and the bonding symbol used to denote a mixture of R and S configurations
about a given asymmetric center.
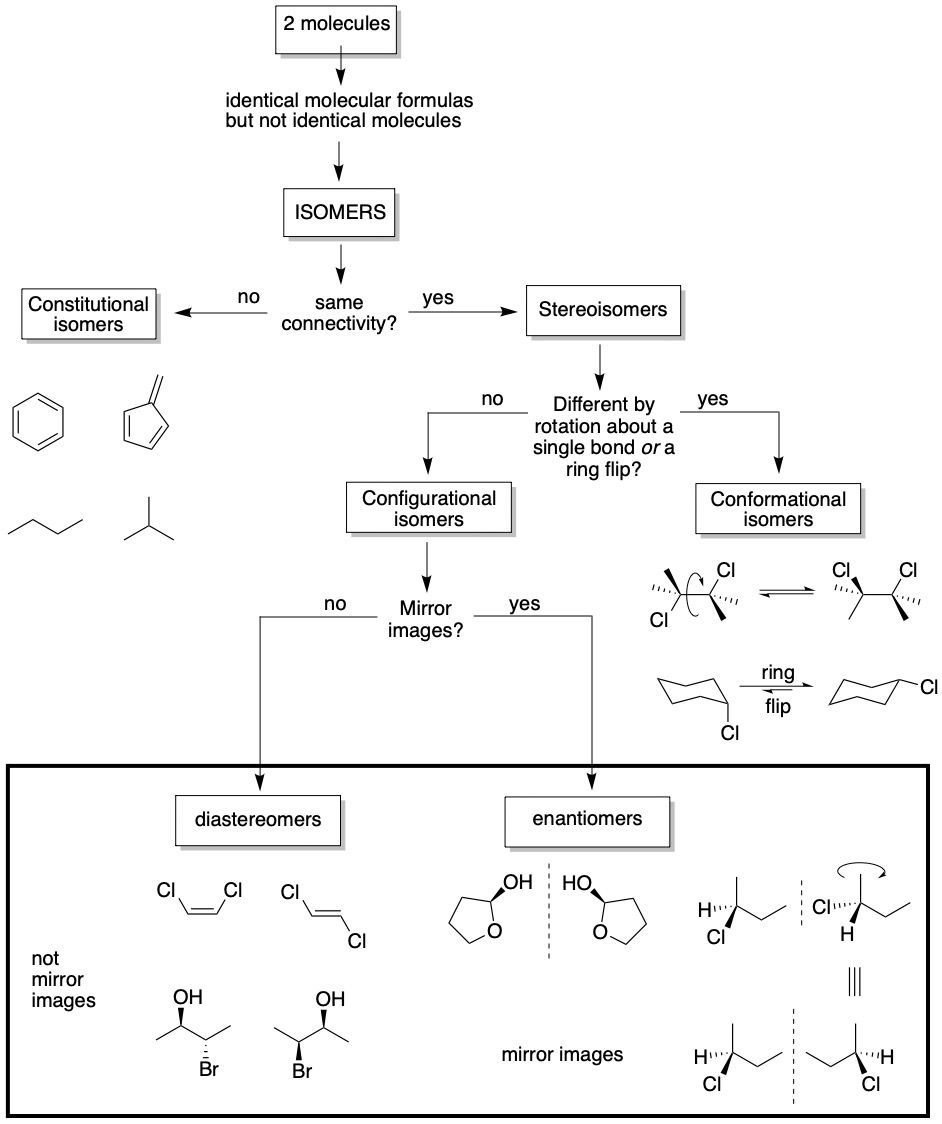
Definitions of terms
stereoisomers: two (or more) molecules with the same number and type of atoms with the same connectivity yet are still different compounds (non-superposable).
stereocenter: an atom with the property that interchanging any two of its substituents produces a different stereoisomer.
asymmetric center (chiral center): any atom with four chemically different substituents attached to it. An asymmetric center is a type of stereocenter, but not all stereocenters are asymmetric centers.
chiral, chirality: the geometric property of some molecules wherein a compound is non-superposable on its mirror image.
enantiomers: configurational stereoisomers that are mirror images of one another.
enantiomeric: the relationship between a pair of molecules which are enantiomers.
enantiopic/enantiomorphic: the relationship between two mirror image groups or atoms within a single molecule.
diastereomers: configurational stereoisomers that are not mirror images of one another.
diastereomeric: the relationship between a pair of molecules which are diastereomers.
diastereotopic/diastereomorphic: the relationship between two constitutionally identical, non-mirror image groups or atoms within a single molecule.
racemic mixture: a 50:50 mix of stereoisomers.
epimer: each of two isomers with different configurations of atoms around only one of several stereogenic carbon atoms present.
anomer: An epimer is a stereoisomer that differs in configuration at any single stereocenter. An anomer is an epimer at the hemiacetal/acetal carbon in a cyclic saccharide, an atom called the anomeric carbon.
to superpose: to place one geometric figure in the space occupied by another so that the two figures coincide throughout their whole extent (i.e., are identical).
to superimpose: to impose, place, or set one figure over, above, or on another figure; to put or join as an addition. Two non-identical figures can be superimposed.
64.01 CONFIGURATIONAL ISOMERS: ENANTIOMERS AND DIASTEREOMERS
Configurational isomers are two or more molecules that do not differ in bonding arrangement of the atoms or the rotation of the bonds or ring inversion, but in some other way. There are two kinds of configurational isomers: enantiomers and diastereomers. Enantiomer comes from the Greek enantios, ‘opposite’, and meros, ‘part’. Dia is a common prefix with multiple meanings, including ‘throughout’, ‘across’, and pertinently ‘opposite’.
Either a set of enantiomers or a set of diastereomers are non-superposable molecules, which means that there is no possible orientation in which all the atoms in both molecules will superpose (or ‘line up’ with one another). Enantiomers are a pair of molecules that are non-superposable mirror images while diastereomers are non-superposable molecules that are not mirror images. We will see later that these ‘certain locations’ are associated with stereocenters.
A note on superposable vs superimposable:
In modern organic chemistry parlance, the term ‘superimposable’ has come to have the same meaning as ‘superposable’, namely “two or more objects that can be placed in the same space and be the same throughout their whole extent.” While superimposable is by far the more common term, this author prefers the term superposable over superimposable.
to superpose: “to place an object on/above another, especially so that they are identical.”
to superimpose: “to place an object on/above another, especially so that both remain visible”.
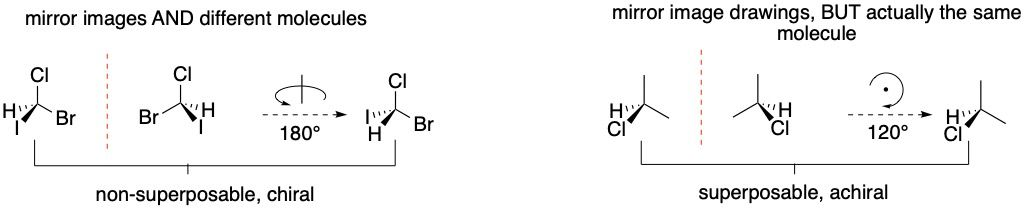
16.02 How to distinguish enatiomers and diastereomers
To determine if two molecular representations are the same molecule, enantiomers, or diastereomers, one must rotate line structures. A few examples of flipping/rotating structures are shown.
Your goal is to move a given substituent on both images into the same location and then compare the images.
- If the two images are superposable (identical), then your analysis is done.
- If they not superposable, then flip rotate until you can compare the images as mirror images (enantiomers).
- If the two images are not the same and they not mirror images, then they are diastereomers.
Flipping and rotating molecules in your mind, particularly rotation about single bonds, is quite challenging. At first, sketch each step of the process. Only after you develop proficiency should you attempt flipping/rotation in your mind without the aid of redrawing.


64.02.1 Are the following pairs of structures superposable or not? If they are superposable, then what is their relationship? If they are non-superposable, then what is their relationship (enantiomeric or diastereomeric)?


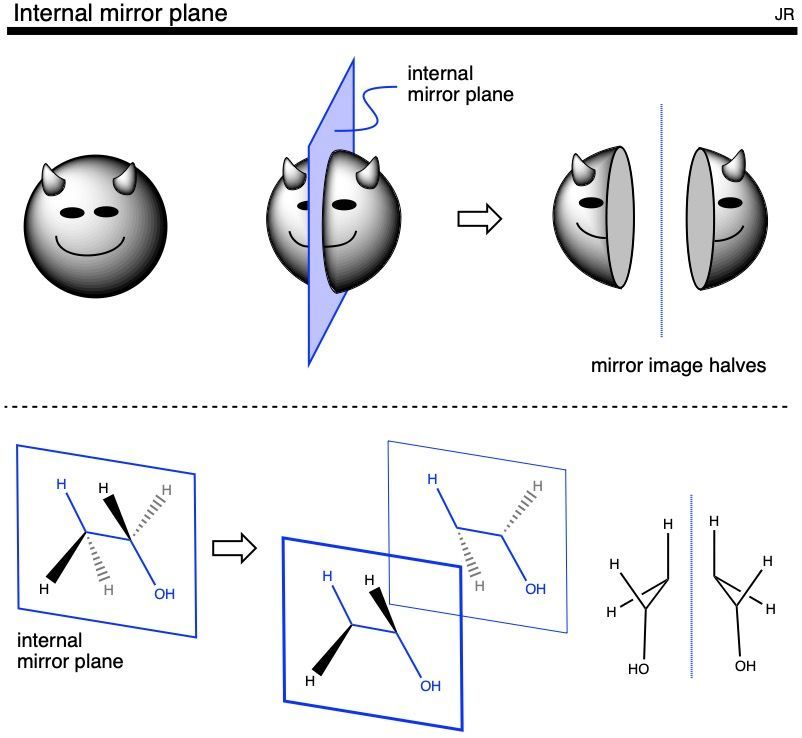
64.03 CHIRALITY AND INTERNAL MIRROR PLANES
A compound that cannot be superposed upon its mirror image structure is a chiral compound. Conversely, molecules that are superposable upon there mirror image structure are achiral compounds. The mirror image structure of a chiral molecule will always be a different molecule, while the mirror image structure of an achiral molecule will be the same molecule, just viewed from a different perspective. The term chiral comes from the Greek word for ‘hand’, and you will often hear chiral explained in terms of ‘handedness’.
The technique of redrawing a molecule as its mirror image to determine the molecule’s chirality / achirality is time-consuming and cumbersome task. A more efficient technique is identifying any internal mirror planes. An internal mirror plane is an imaginary two-dimensional plane that bisects a molecule yielding mirror image halves. It is common to conflate ‘the mirror image of a molecule’ with the hypothetical mirror image halves of a single molecule produced by cutting the strucutre in half: These two concepts are not the same. For example: a molecule which could have a distinct, mirror image molecular partner is an example of a chiral compound, and it turns out that these structures have no internal mirror plane. A molecule that does possess an internal mirror plane simply possesses mirror image parts of itself and this means the compound is achiral. Another common term for internal mirror plane is plane of symmetry. The power of identifying the presence (or absence) of internal mirror planes is that one can determine the chirality/achirality of a compound without having to spend the time redrawing the compound as the mirror image and comparing the two structures directly. Importantly there are structures with no obvious internal mirror planes that are actually achiral, but these are rarely invoked at the undergraduate level; see ‘Inversion center’ section below.
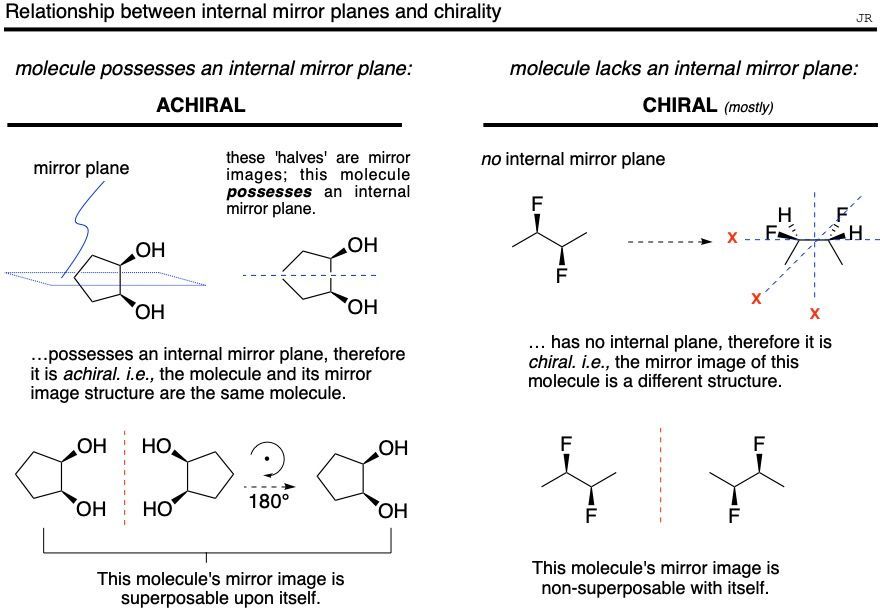
- Any structure that possesses an internal mirror plane is achiral, and it could not possibly be part of an enantiomeric pair. i.e., the molecule’s mirror image is simply itself.
- For the most part, a compound with no internal mirror plane is chiral, and the compound’s mirror image structure would be non-superposable. i.e., the molecule and its mirror image structure would be different compounds (enantiomers). Exceptions to this observation are described in the ‘inversion center section’.
64.03.1 Determine if each compound below possesses an internal mirror plane plan or lacks an internal mirror plane. If there is a plane, sketch it on the molecule.


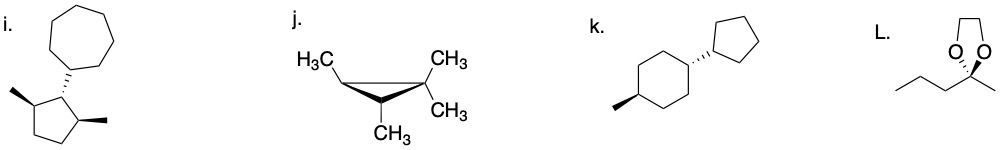
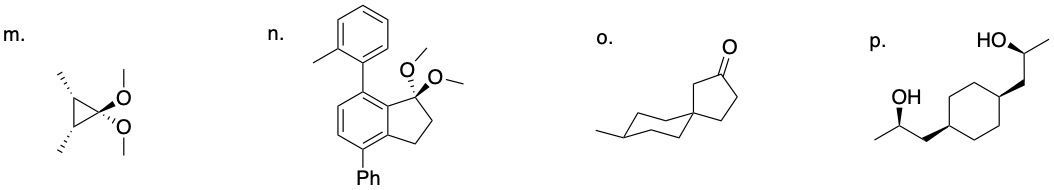
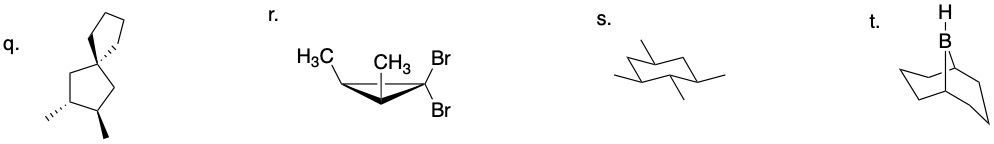
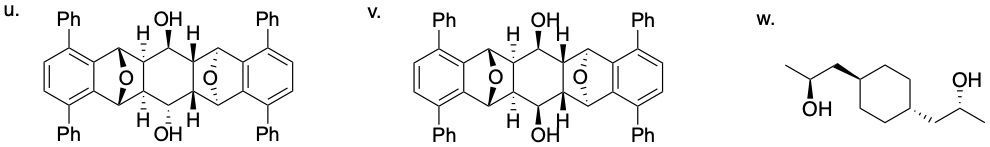
64.01 CONFIGURATIONAL ISOMERS: ENANTIOMERS AND DIASTEREOMERS
Configurational isomers are two or more molecules that do not differ in bonding arrangement of the atoms or the rotation of the bonds or ring inversion, but in some other way. There are two kinds of configurational isomers: enantiomers and diastereomers. Enantiomer comes from the Greek enantios, ‘opposite’, and meros, ‘part’. Dia is a common prefix with multiple meanings, including ‘throughout’, ‘across’, and pertinently ‘opposite’.
Either a set of enantiomers or a set of diastereomers are non-superposable molecules, which means that there is no possible orientation in which all the atoms in both molecules will superpose (or ‘line up’ with one another). Enantiomers are a pair of molecules that are non-superposable mirror images while diastereomers are non-superposable molecules that are not mirror images. We will see later that these ‘certain locations’ are associated with stereocenters.
A note on superposable vs superimposable:
In modern organic chemistry parlance, the term ‘superimposable’ has come to have the same meaning as ‘superposable’, namely “two or more objects that can be placed in the same space and be the same throughout their whole extent.” While superimposable is by far the more common term, this author prefers the term superposable over superimposable.
to superpose: “to place an object on/above another, especially so that they are identical.”
to superimpose: “to place an object on/above another, especially so that both remain visible”.

